SOOPR™
A Rescue Agent Designed Specifically for Fentanyl Overdose

Fentanyl Is Killing Us
After finding her son unresponsive in his room, Megan races to the phone to call 911. They live in rural America, a long way from the closest hospital and emergency responders. However, Megan remains hopeful as her mind flashes back to the two previous times that she used Narcan to rescue her son, who began abusing opioids only two years ago after a sports injury.
This time is different. After administering the first dose of Narcan, her son begins to come around, but within minutes, he is unresponsive. Luckily, Megan has a
second dose of Narcan to give him, but even after administering it, she can tell she’s losing him. Megan has heard about fentanyl on the news but didn’t realize that current rescue agents aren’t always effective against such potent synthetic opioids. First responders arrive and save her son’s life using additional doses of Narcan.
Sadly,because the respiratory depression caused by fentanyl starved his brain of oxygen, he suffers a toxic brain injury. No longer able to walk, talk or feed himself, he will require life-long care.
Watching helplessly as someone you love falls victim to overdose is a horrifying scene, but with the proliferation of fentanyl into the American drug scene, it has tragically become a common one.
Fentanyl: An Evolving Nightmare
Fentanyl is the #1 cause of death for Americans aged 18-45.
This highly potent synthetic opioid has flooded U.S. streets, and is now found in nearly every illicit drug supply. Fentanyl overdose claimed the lives of 40,010
Americans ages 18-45—more than car accidents, suicide, COVID-19, and cancer. In total, 71,000 people died in the U.S. from synthetic opioid poisoning in 2021, up from 57,000 in 2020. That’s 200 people a day. Imagine a Boeing 737 crashing every single day, and you will get a sense of the magnitude of fatalities fentanyl leaves in its wake.
Sizing Up The Enemy: Why Is Fentanyl So Dangerous?
Unmatched Potency
Fentanyl and its analogs are between 50 and 5,000 times more potent than heroin. People often are unaware that the drugs they are taking are laced with fentanyl and overdose as a result.
Deadly Analogs
Fentanyl is just one member of an emerging class of deadly illicit synthetic opioids. Knowledge of how to create and mass produce increasingly po- tent analogs of fentanyl is common in illicit drug circles. Twelve known iterations already exist.
Onset + Duration
The onset of fentanyl overdose occurs rapidly, and the duration is significantly longer than that of other drugs, leading to re-narcotization (return of overdose symptoms) after rescue with short- acting reversal agents like Narcan.
Why Are Current Solutions Failing?
The common rescue agent for overdose is naloxone (Narcan nasal spray), adminis- tered by a first responder or friend/family member. Narcan blocks the opioid recep- tors in the brain, so they can no longer uptake drugs like fentanyl and heroin. A single dose works well against overdoses caused by certain opioids (e.g., heroin, oxycodone), but against long-acting synthetic opioids like fentanyl, Narcan has two critical shortcomings:
1) Ineffective Against Fentanyl’s Potency
Narcan was not designed with the potency of fentanyl in mind, and it falls short. To be effective, it often requires multiple doses, which are not typically available to friends and family. For these potent synthetic opioids, a more effective rescue agent is needed.
2) Short Window of Effectiveness
Fentanyl’s lethal effect on breath- ing lasts significantly longer than other opioids, causing severe brain injury or death even after admin- istering existing rescue agents.
Further, existing rescue agents do not provide a meaningful interven- tion window in which friends and family could get their loved one on a road to recovery.
What is Needed? A Viable Counter-Attack Against Fentanyl.
Elysium’s mission is to reduce suffering from opioid overdose, opioid use disorder and severe pain. The tragic rise in synthetic opioid deaths is clear evidence that existing rescue agents are no match for these potent opioids.
Consequently, we set out to engineer a rescue agent that provides loved ones of OUD (opioid use disorder) sufferers and brave first responders with the peace of mind that comes from knowing they are equipped to save a life and help someone on the road to recovery.

Introducing Elysium’s Solution:
SOOPR™
(Synthetic Opioid Overdose Prevention and Rescue)
We interviewed first responders and OUD treatment professionals about what an effective solution should look like. We used their requirements as a north star in engineering SOOPR (Synthetic Opioid Overdose Prevention and Rescue). SOOPR is a long-acting rescue agent, created by a proprietary molecular modification of Nalmefene, a known, effective opioid antagonist.
Simple Dosing Process
We interviewed first responders and OUD treatment professionals about what an effective solution should look like. We used their requirements as a north star in engineering SOOPR (Synthetic Opioid Overdose Prevention and Rescue). SOOPR is a long-acting rescue agent, created by a proprietary molecular modification of Nalmefene, a known, effective opioid antagonist.
Long-Acting Solution
SOOPR is designed to block opioid receptors for 18-24 hours, providing an infusion-like delivery of nalmefene. This reduces risk of renarcotization, or the return of overdose symptoms, and, thus, significantly reduces the likelihood of death or toxic brain injury.
Meaningful Intervention Window
Friends and family will have the time to help their loved one on the road to recovery. SOOPR could provide an effective bridge to medically-assisted OUD therapy, providing OUD sufferers with the best chance at recovery.
On a road of suffering, SOOPR is the on-ramp toward hope.
People with OUD are barreling down a road of suffering toward early death or lasting brain injury. SOOPR provides a life-changing detour, an on-ramp to hope. A SOOPR rescue could be the turning point, granting someone a second chance at life, and the time to get the help they need.
Who Benefits?
OUD sufferers get a second chance at life, and their best chance of recovery.
Families of people with OUD can have peace of mind, knowing their loved one’s life can be saved.
First responders can use SOOPR as a 24-hour shield against acci- dentally inhaling fentanyl, which can be lethal.
Your Opportunity
Within 5 years and an investment of ~$50M Elysium could complete the FDA requirements to launch SOOPR and fulfill its mission to reduce suffering from opioid overdose and opioid use disorder.
Millions of Americans are suffering, and we are losing the battle against fentanyl. With your support, we can save thousands of lives each year and provide OUD sufferers their best chance at recovery.
To learn more about joining the fight, please contact:
Greg Sturmer
President and CEO
(650) 464-6175
SMART™ Opioids with Unprecedented Protection Against Abuse and Oral Overdose
(Results of Human Proof of Concept Study)
T. Jenkins, Ph.D*., Lynn R. Webster, MD✣, Leela Vrishabhendra, MD✦ and A. Greg Sturmer*
*Elysium Therapeutics, Inc.
✣Executive Vice President Scientific Affairs, Dr. Vince Clinical Research, Overland Park, Kansas
✦Senior Medical Director, Medpace, Inc. Cincinnati, Ohio
Background
Elysium is developing a novel class of SMART™ Opioids to reduce suffering from fatal opioid overdose, opioid-use disorder, and moderate-to-severe acute pain that cannot be effectively treated with non-opioid analgesics.
The vast majority (>90%) of the abuse of short-acting prescription opioids is via co-ingestion of supratherapeutic doses (Ref. 1). This is reinforced by two key factors:
A dosing regimen that necessitates a high number of prescribed tablets resulting in multiple opportunities to reinforce abuse
The incentive for abusers to ingest supratherapeutic doses for increased euphoric effects, often resulting in opioid use disorders and potentially lethal overdoses.
Elysium’s SMART™ Opioids
Elysium’s SMART opioid prodrugs leverage our O2P (Oral Overdose Protected) technology — novel prodrugs designed to effectively mitigate non-oral and oral abuse while safely delivering an FDA approved opioid agonist.
Elysium’s O2P hydrocodone offers a highly sought-after and unprecedented product profile, including:
Safe and well-tolerated
Efficient delivery of therapeutic hydrocodone exposures at prescribed doses
Attenuation of hydrocodone exposures (e.g., Cmax) when supratherapeutic doses are ingested
Hydrocodone pharmacokinetics consistent with once-daily dosing
Protection against non-oral abuse and overdose (i.e., hydrocodone not released unless ingested)
Robust chemical stability that renders O2P molecules highly tamper-resistant.
O2P opioid prodrug technology is broadly applicable to all currently prescribed oral opioid agonists.
O2P Mechanism of Action
O2P hydrocodone prodrug molecules comprise (i) a trypsin-activated opioid delivery subunit that efficiently releases therapeutic levels of hydrocodone only when exposed to the digestive enzyme trypsin in the lumen of the small intestine, and (ii) a trypsin inhibitor subunit that progressively inhibits the trypsin-mediated release of hydrocodone when supratherapeutic doses are ingested.
Human Proof-of-Concept Study
Elysium conducted a phase 1, randomized, open-label, 2-part study to evaluate the safety and pharmacokinetics of oral O2P Hydrocodone vs. hydrocodone bitartrate (HCBT) in healthy adult subjects under fasted conditions under naltrexone blockade (O2P-001).
Key objectives of the study were to:
Evaluate the safety, tolerability, and pharmacokinetics of oral O2P Hydrocodone relative to a HCBT comparator following single oral doses in healthy adult subjects.
Demonstrate reduced maximum plasma exposures (Cmax) of hydrocodone delivered from escalated oral doses of O2P Hydrocodone relative to an escalated comparator dose of HCBT (i.e. oral overdose protection).
Identify appropriate doses of oral O2P Hydrocodone for subsequent clinical studies.
Population
The population for this study included male and female subjects, 18 to 55 years of age, inclusive, who were in general good health.
Study Design
93 healthy adult subjects were enrolled for the study with each subject participating in 1 treatment period. Six subjects were included in each treatment period.
Safety assessments included incidence of adverse events (AEs), physical examination findings, vital signs, electrocardiogram (ECG) parameters, and clinical laboratory tests.
For safety, subjects were administered oral naltrexone pre- and post-dose for each treatment period.
Blood sampling and subsequent PK analyses were performed during the study.
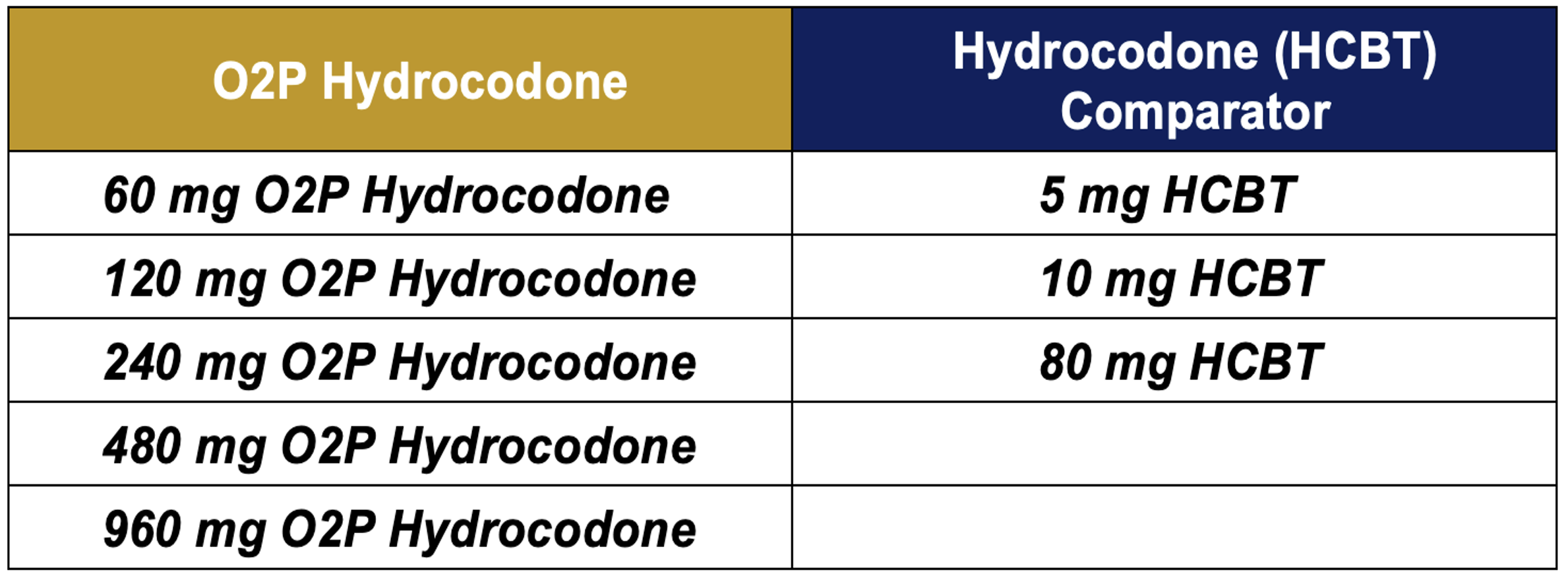
Table 1: Selected Treatments for the O2P-001 Study
Results
Safety
Safety was assessed by evaluating physical examinations, vital signs (BP, heart rate, respiratory rate, oxygen saturation, and oral body temperature), 12-lead ECGs, clinical laboratory parameters (serum chemistry, hematology, and urinalysis), and AEs reported during the study.
In all treatment periods O2P Hydrocodone was generally well tolerated in all subjects with no serious adverse events reported.
Study drug related Treatment-Emergent Adverse Events (TEAEs) occurrence was 19.4% for the entire study population (n=93).
All TEAEs were classified and mild or moderate.
Study Drug Related TEAEs included nausea, emesis abdominal pain/distension, diarrhea, dizziness, headache, presyncope, anxiety, insomnia, and agitation.
There were no clinically significant clinical laboratory values, ECG results, or out-of-range vital signs following treatments with O2P hydrocodone or HCBT.
Pharmacokinetics
Data from the 47 subjects who completed the selected treatments listed in Table 1 were included for pharmacokinetic analysis.
Hydrocodone plasma concentration vs. time data for the 60 and 120 mg doses of O2P hydrocodone and the 5 and 10 mg HCBT comparators are presented in Figure 1.
Based on comparable Cmax values, the 5 mg HCBT vs. 60 mg O2P hydrocodone doses, as well as the 10 mg HCBT vs. the 120 mg O2P hydrocodone doses represent Hydrocodone Equivalent Doses (HEDs).
Figure 1: Plasma Hydrocodone Concentrations vs. Time for 60 and 120 mg Doses of O2P Hydrocodone vs. the 5 and 10 mg HCBT Comparator Doses
Oral Overdose Protection
Dose escalations of O2P hydrocodone and HCBT were conducted to evaluate the extent of oral overdose protection exerted with O2P hydrocodone.
Escalating O2P hydrocodone doses of 60, 120, 240, 480 and 960 mg were compared to 5, 10, and 80 mg doses of HCBT (Table 1).
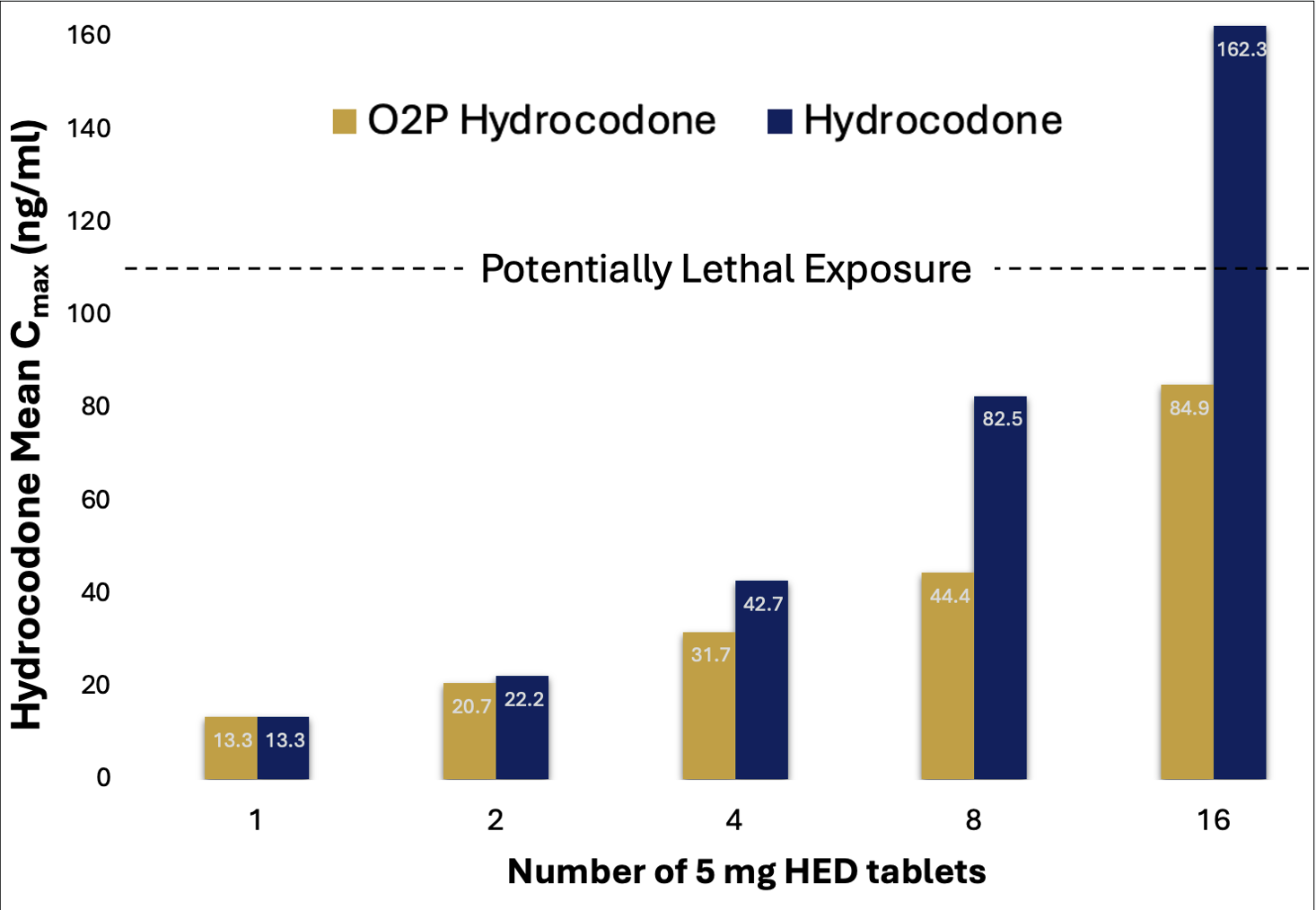
Mean hydrocodone Cmax values for escalating doses of O2P hydrocodone and HCBT are presented in Figure 2.
For comparison purposes, the mean HCBT Cmax values depicted in Figure 2 were proportionally adjusted so that the Cmax values for the 60 mg O2P hydrocodone and the 5 mg dose for HCBT were normalized.
Mean Cmax values for O2P hydrocodone and HCBT in Figure 2 are benchmarked against a potentially lethal hydrocodone plasma exposure of ~110 ng/ml.3
Cmax values from 8- and 16-fold doses for O2P hydrocodone were significantly reduced (~50%) vs. the 8- and 16-fold doses of HCBT.
The Cmax value from highest dose of HCBT exceeded a potentially lethal exposure, while the Cmax value produced with highest dose of O2P hydrocodone did not. (Ref. 3)
Figure 2: Mean Cmax values for O2P hydrocodone and HCBT vs. dose
Cmax values for the 4- and 8-fold doses of HCBT were extrapolated from the Cmax vs. dose curve established using the 5, 10 and 80 mg HCBT doses evaluated in the study. Hydrocodone Cmax values are normalized to be equivalent at the single dose level.
Conclusions
Key Benefits of O2P Hydrocodone Vs. Currently Prescribed Hydrocodone:
Significant Reduction in The Number of Prescribed Doses Mitigates Misuse, Abuse and Diversion
The sustained exposure of hydrocodone demonstrated by O2P hydrocodone (Figure 1) is consistent with a once-daily dosing regimen.
Consequently, the number of prescribed tablets for O2P hydrocodone is significantly reduced vs. currently prescribed hydrocodone (Figure 3).
Figure 3: Tablets per Rx
Limits the Number of Euphoric Experiences that Serve to Initiate and Reinforce Abuse Behavior
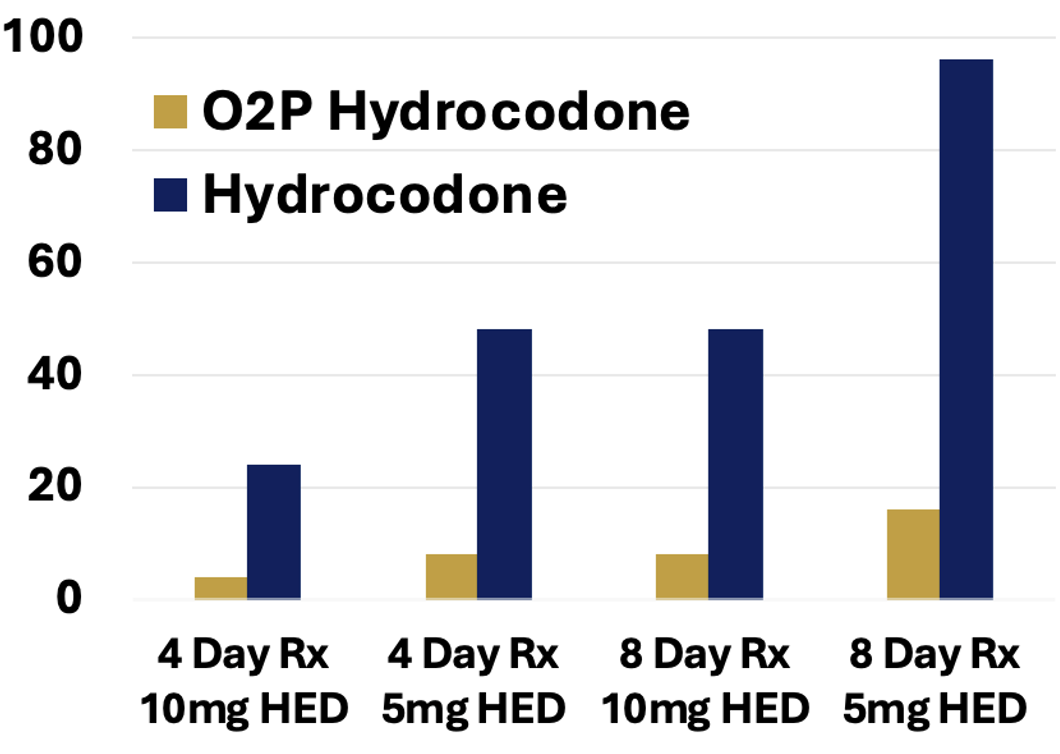
The PK/PD relationship for hydrocodone drug liking has been well characterized in multiple human abuse liability (HAL) studies. A 20 mg dose of hydrocodone has been demonstrated to produce a euphoric effect that is well-liked and may reinforce abuse. (Ref. 2)
Based on the attenuated hydrocodone exposures with O2P hydrocodone at supratherapeutic doses, a dose of 480 mg of O2P hydrocodone would be required to achieve a comparable experience. Consequently, the number of euphoric experiences) for a O2P hydrocodone prescription is dramatically reduced vs. currently prescribed hydrocodone (Figure 4).
Figure 4: 20 mg HEDs per Rx
Significantly Reduces the Risk of Fatal Overdose
The demonstrated oral overdose protection which reduces hydrocodone exposures when supratherapeutic doses are ingested in concert with a once-daily dosing regimen (i.e., fewer tablets per prescription) serve to significantly reduce the risk of potentially fatal overdose.3
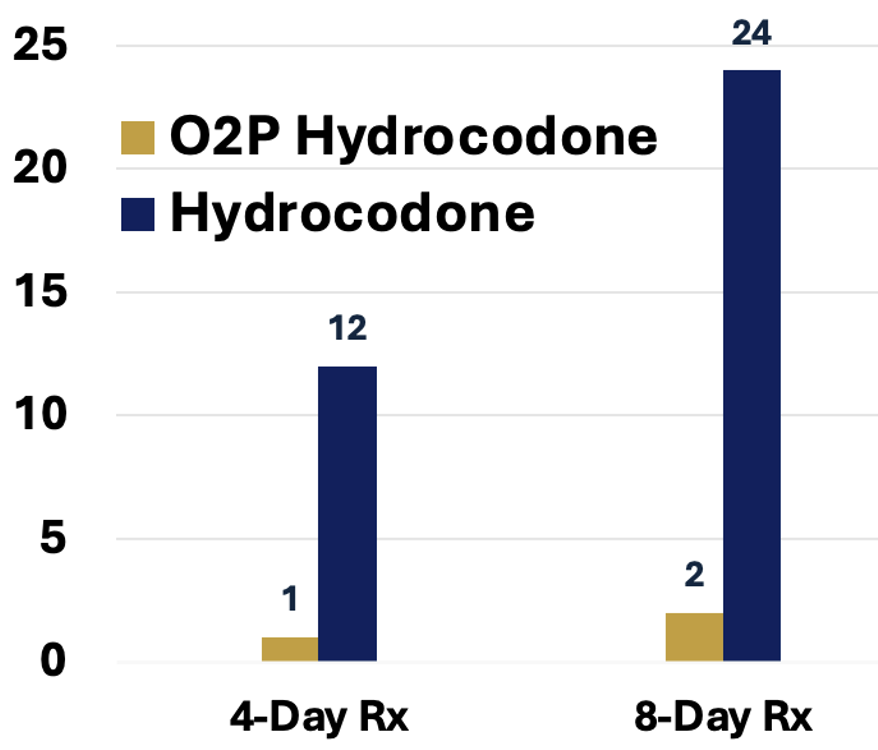
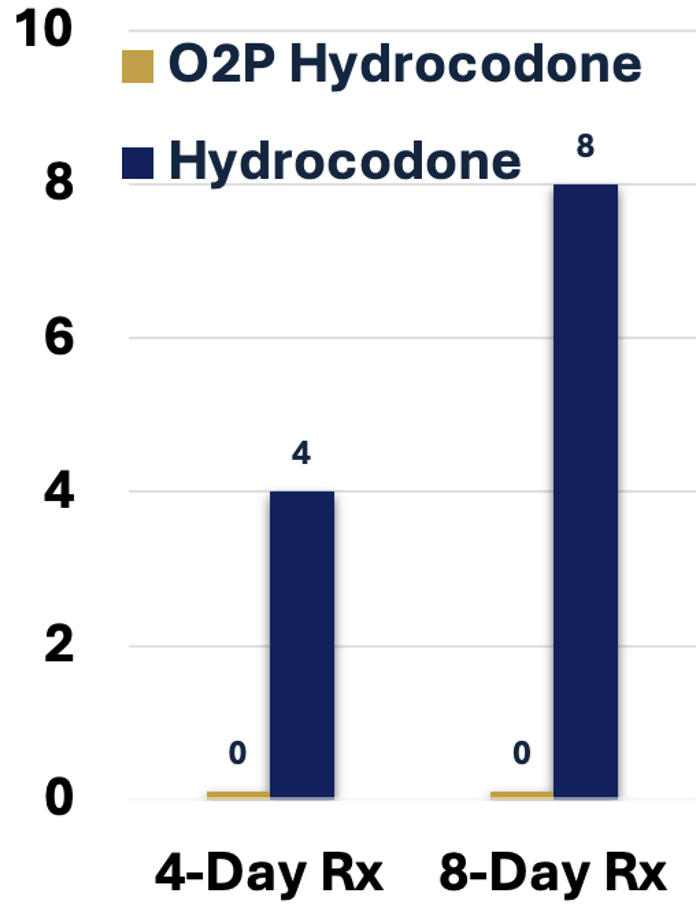
A 4- or 8-day prescription of currently prescribed hydrocodone contains 4- or 8-fold the lethal dose of hydrocodone, respectively (Figure 5).
In stark contrast, a 4- or 8-day prescription of O2P hydrocodone cannot produce a lethal plasma exposure of hydrocodone even if the entire prescription is taken as a single dose (Figure 6).
Figure 5: Lethal Doses per Rx
Figure 6: Cmax per Rx vs. Potentially Lethal Cmax
References:
Ref. 1
Gasior, Maciej; Bond, Mary; Malamut, Richard (2016): Routes of abuse of prescription opioid analgesics. A review and assessment of the potential impact of abuse-deterrent formulations. In Postgraduate medicine 128 (1), pp. 85–96.
Ref. 2
Walsh SL, Nuzzo PhydromorphoneA, Lofwall MR, Holtman JR Jr. The relative abuse liability of oral oxycodone, hydrocodone and assessed in prescription opioid abusers. Drug Alcohol Depend. 2008 Dec 1;98(3):191-202.
Ref. 3
Molina DK, Hargrove VM. What is the lethal concentration of hydrocodone? A comparison of postmortem hydrocodone concentrations in lethal and incidental intoxications. Am J Forensic Med Pathol. 2011 Jun;32(2):108-11.